Featured Articles
2023-04-25
[2023-04-25 ~
]
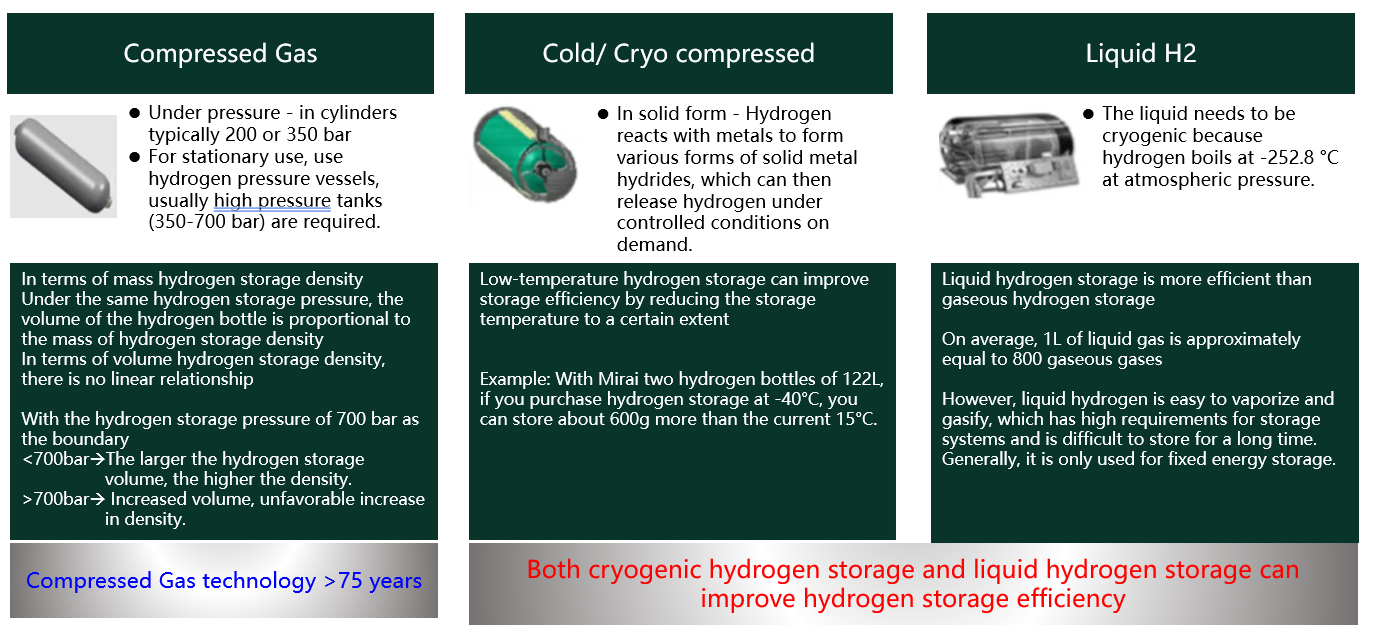
How is hydrogen stored?
The Safety of New Energy Vehicle - Hydrogen Storage Method
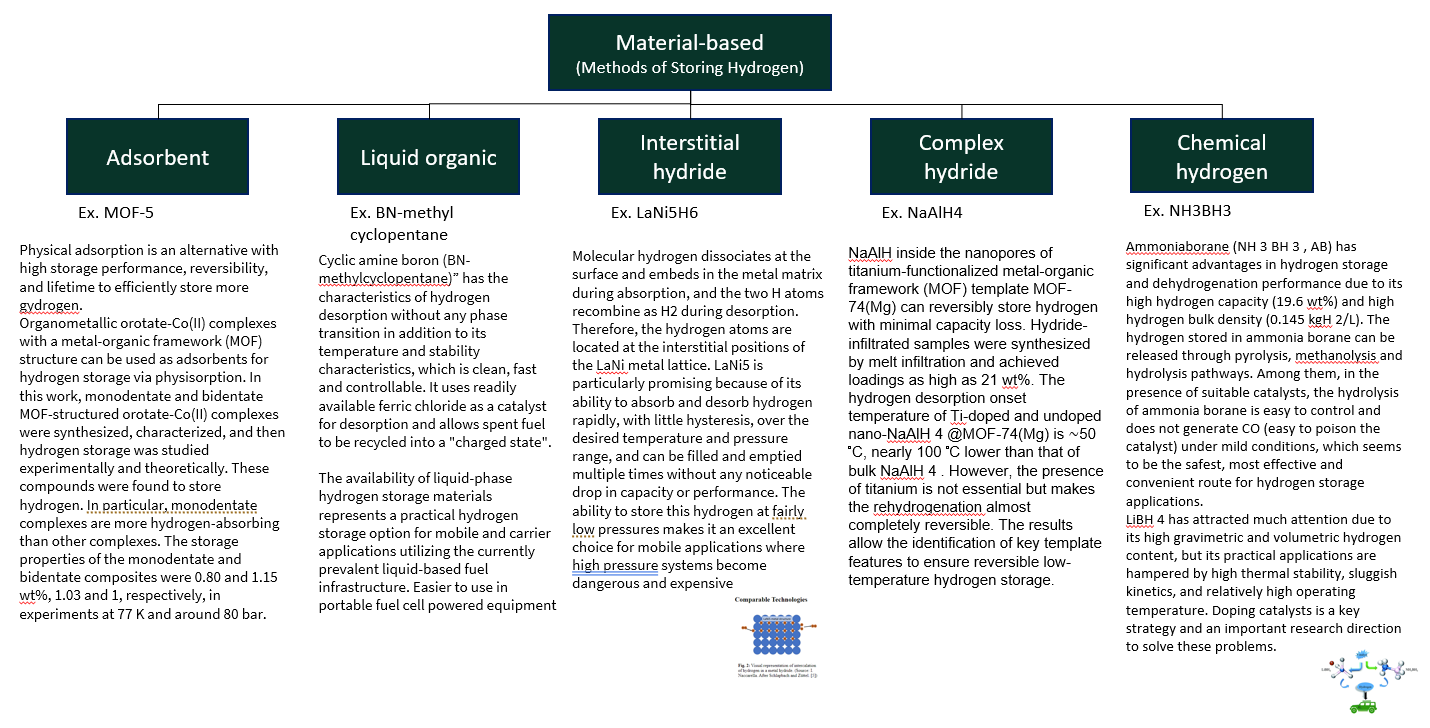 Material-based(Methods of Storing Hydrogen)
Material-based(Methods of Storing Hydrogen)Adsorbent:Ex. MOF-5
Physical adsorption is an alternative with high storage performance, reversibility, and lifetime to efficiently store more gydrogen.
Organometallic orotate-Co(II) complexes with a metal-organic framework (MOF) structure can be used as adsorbents for hydrogen storage via physisorption. In this work, monodentate and bidentate MOF-structured orotate-Co(II) complexes were synthesized, characterized, and then hydrogen storage was studied experimentally and theoretically. These compounds were found to store hydrogen. In particular, monodentate complexes are more hydrogen-absorbing than other complexes. The storage properties of the monodentate and bidentate composites were 0.80 and 1.15 wt%, 1.03 and 1, respectively, in experiments at 77 K and around 80 bar.
Liquid organic:Ex. BN-methyl cyclopentane
Cyclic amine boron (BN-methylcyclopentane)” has the characteristics of hydrogen desorption without any phase transition in addition to its temperature and stability characteristics, which is clean, fast and controllable. It uses readily available ferric chloride as a catalyst for desorption and allows spent fuel to be recycled into a "charged state".
The availability of liquid-phase hydrogen storage materials represents a practical hydrogen storage option for mobile and carrier applications utilizing the currently prevalent liquid-based fuel infrastructure. Easier to use in portable fuel cell powered equipment
Interstitial hydride:Ex. LaNi5H6
Molecular hydrogen dissociates at the surface and embeds in the metal matrix during absorption, and the two H atoms recombine as H2 during desorption. Therefore, the hydrogen atoms are located at the interstitial positions of the LaNi metal lattice. LaNi5 is particularly promising because of its ability to absorb and desorb hydrogen rapidly, with little hysteresis, over the desired temperature and pressure range, and can be filled and emptied multiple times without any noticeable drop in capacity or performance. The ability to store this hydrogen at fairly low pressures makes it an excellent choice for mobile applications where high pressure systems become dangerous and expensive
Complex hydride:Ex. NaAlH4
NaAlH inside the nanopores of titanium-functionalized metal-organic framework (MOF) template MOF-74(Mg) can reversibly store hydrogen with minimal capacity loss. Hydride-infiltrated samples were synthesized by melt infiltration and achieved loadings as high as 21 wt%. The hydrogen desorption onset temperature of Ti-doped and undoped nano-NaAlH 4 @MOF-74(Mg) is ∼50 °C, nearly 100 °C lower than that of bulk NaAlH 4 . However, the presence of titanium is not essential but makes the rehydrogenation almost completely reversible. The results allow the identification of key template features to ensure reversible low-temperature hydrogen storage.
Chemical hydrogen:Ex. NH3BH3
Ammoniaborane (NH 3 BH 3 , AB) has significant advantages in hydrogen storage and dehydrogenation performance due to its high hydrogen capacity (19.6 wt%) and high hydrogen bulk density (0.145 kgH 2/L). The hydrogen stored in ammonia borane can be released through pyrolysis, methanolysis and hydrolysis pathways. Among them, in the presence of suitable catalysts, the hydrolysis of ammonia borane is easy to control and does not generate CO (easy to poison the catalyst) under mild conditions, which seems to be the safest, most effective and convenient route for hydrogen storage applications.
LiBH 4 has attracted much attention due to its high gravimetric and volumetric hydrogen content, but its practical applications are hampered by high thermal stability, sluggish kinetics, and relatively high operating temperature. Doping catalyst is a key strategy and an important research direction to solve these problems.
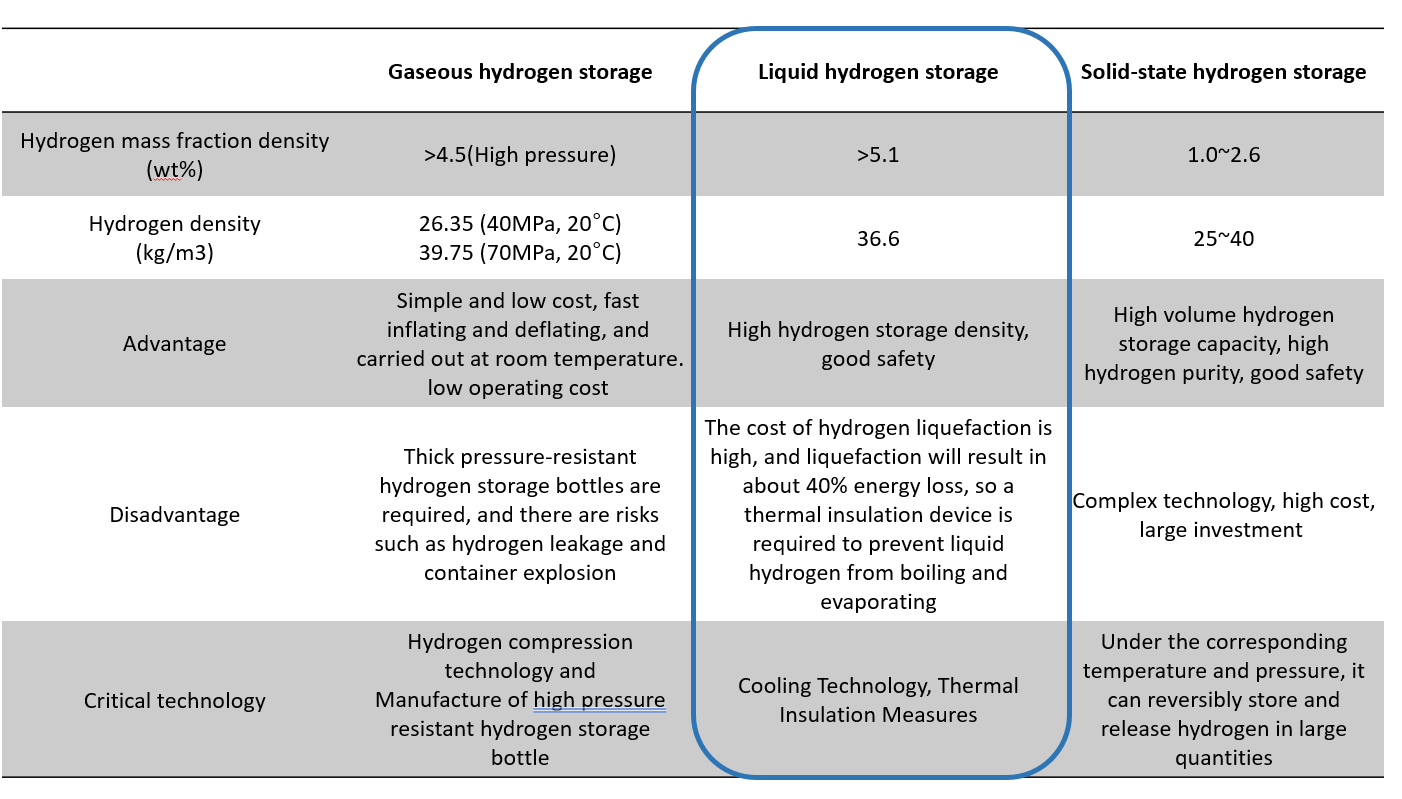
The Critical Technology of New Energy Vehicle - Hydrogen Storage Method